Vision
A world in which people in need in low- and middle-income countries (LMICs) have rapid access to effective and affordable medical treatments and health technologies.
Mission
Our mission is to increase equitable access to innovative medicines and other health technologies through public health-oriented voluntary licensing and technology transfer.
Mandate
MPP’s mandate is to accelerate access to affordable quality treatments for people living with HIV, hepatitis C and tuberculosis, as well as HIV-associated co-morbidities. Since 2018, MPP has expanded its mandate to other patented essential medicines on the World Health Organization (WHO)’s Model List of Essential Medicines (EML) as well as medicines with strong potential for future inclusion on the EML. In 2020, we temporarily expanded MPP’s mandate to include COVID-19 treatments. In 2021, MPP expanded its mandate into the licensing of technology with an initial focus on COVID-19 vaccines and pandemic preparedness.
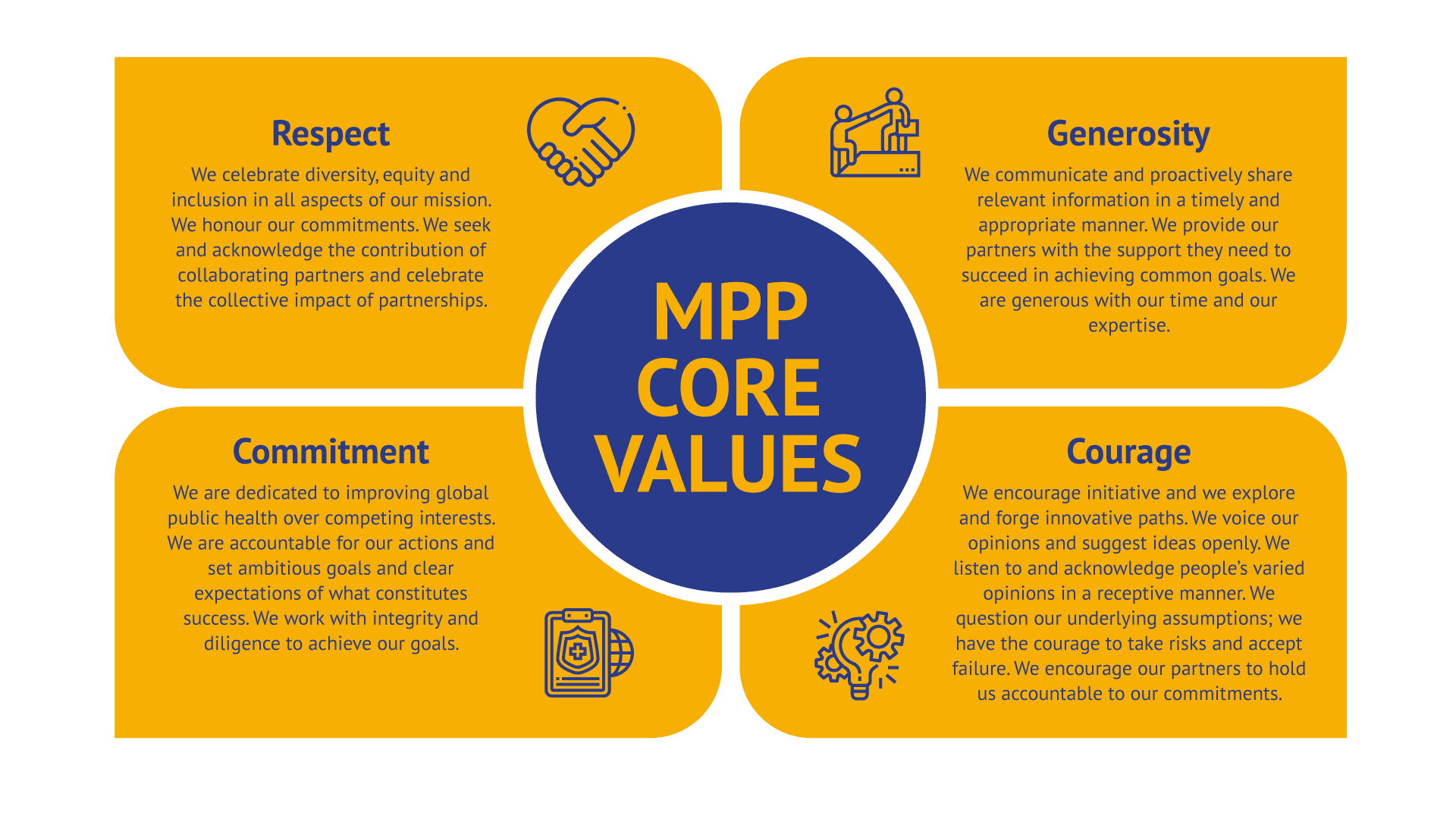
MPP Culture and Values
At MPP we are committed to making sure novel quality medicines are available and affordable in the shortest of time for those who would otherwise be left behind.
In order to accomplish our mission, we strive for excellence in our work and foster a positive organisational culture through transparency and open communication. We hold ourselves accountable to the highest standards, respecting individuality and encouraging innovation. We offer support to our partners as we share our learnings with the aim of accelerating access to treatment. In all what we do, we all do our best to stretch our own ability and capacity.
MPP in Numbers
patent holders with MPP signed agreements
generic manufacturers and product developers have sublicences from MPP
History
MPP was established in 2010 by Unitaid. Its vision was that non-exclusive voluntary licensing through a public health agency would enable more people in LMICs to access affordable treatments. Since then, MPP has succeeded in revolutionising the access landscape for new and essential treatments in resource-limited settings.
MPP started its work in HIV, where there were access gaps in relation to several new antiretroviral medicines. There needed to be an established mechanism for licensing under public health-oriented terms and conditions that would enable manufacturers to develop quality-assured generic products, and make them available where people could not have accessed them otherwise.
Today, more people can access better HIV treatments at affordable prices, and much faster than ever before. For instance, millions of people around the world have now gained access to dolutegravir (DTG) and DTG-based treatments at a much lowered price. You can read more on MPP’s contribution to making DTG available to people who need it most.




